|
History > 2011 > USA > Health (II)
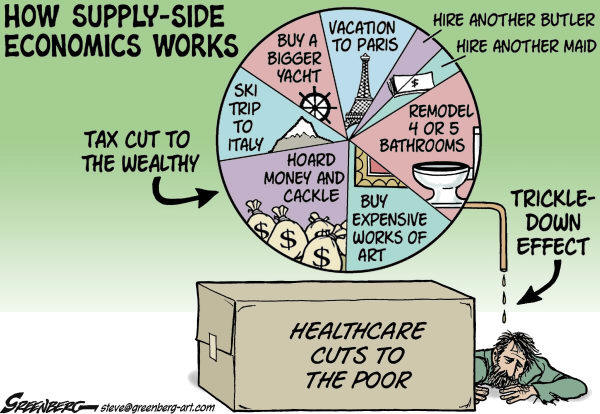
Steve Greenberg
The Ventura County Star
CA
Cagle
21 April 2011
Drugs and Profits
May 24, 2011
The New York Times
By FREDERICK C. TUCKER Jr.
Fredericksburg, Va.
LAST year the Food and Drug Administration rescinded approval of the drug
Avastin for treating breast cancer patients, prompting a firestorm of criticism.
The decision was denounced by some politicians as health care rationing, and by
breast cancer patients who feared that they would be deprived of a drug that
they felt had helped them immensely.
But these criticisms ignore the facts: Avastin was rejected simply because it
didn’t work as it was supposed to, and the F.D.A. should resist the aggressive
campaign by Genentech, the drug’s maker, to get that ruling reconsidered at a
hearing in late June.
Avastin has been on the market for seven years, and combined with other drugs it
is effective in treating, but not curing, some colon, lung, kidney and brain
cancers. It inhibits the development of new blood vessels and in so doing can
starve a growing tumor.
Treating a breast cancer patient with Avastin costs about $90,000 a year, and
Genentech could lose $500 million to $1 billion a year in revenue if the F.D.A.
upholds the ban.
A clinical trial published in 2007 demonstrated that Avastin, when paired with
the chemotherapy drug Taxol, halts the growth of metastatic breast cancer for
about six months longer than chemotherapy alone. Genentech then asked the F.D.A.
for approval of Avastin, combined with Taxol, for use against metastatic breast
cancer.
This halt in tumor growth is known as progression-free survival. But delaying
the worsening of cancer does not necessarily prolong life, and Avastin was not
shown to lengthen patients’ overall survival time. So Genentech argued that the
drug led not to longer life, but to improved quality of life.
In 2007, an F.D.A. advisory committee rejected the application, deciding that
the toxic side effects of Avastin outweighed its ability to slow tumor growth.
The F.D.A., however, overrode the committee and granted what is called
accelerated approval, allowing Avastin to be used pending further study. The
criteria for full approval was that Avastin not worsen overall survival and that
the drug provide clinically meaningful progression-free survival.
To support its case Genentech submitted data from two additional clinical trials
in which Avastin was paired with chemotherapy drugs other than Taxol. Like the
first trial, neither showed a survival benefit. Both showed an improvement in
progression-free survival, though this outcome was much less impressive than in
the original study. In addition to seeking full approval for the Avastin-Taxol
combination, Genentech also asked the F.D.A. to approve the use of Avastin with
the drugs used in these follow-up studies.
Genentech presented progression-free survival as a surrogate for better quality
of life, but the quality-of-life data were incomplete, sketchy and, in some
cases, non-existent. The best that one Genentech spokesman could say was that
“health-related quality of life was not worsened when Avastin was added.”
Patients didn’t live longer, and they didn’t live better.
It was this lack of demonstrated clinical benefit, combined with the potentially
severe side effects of the drug, that led the F.D.A. last year to reject the use
of Avastin with Taxol or with the other chemotherapies for breast cancer.
In its appeal Genentech is changing its interpretation of its own data to pursue
the case. Last year Genentech argued that the decrease in progression-free
survival in its supplementary studies was not due to the pairing of Avastin with
drugs other than Taxol. This year, however, in its brief supporting the appeal,
Genentech argues that the degree of benefit may indeed vary with “the particular
chemotherapy used with Avastin.” In other words, different chemotherapies
suddenly do yield different results, with Taxol being superior. The same data
now generate the opposite conclusion.
Perhaps more troubling is the resort to anecdote in the brief to the F.D.A. and
in the news media. Oncologists recounted their successes, and patients who were
doing well on Avastin argued for its continued approval. But anecdote is not
science. Such testimonials may represent the human voices behind the statistics,
but the sad fact is that there are too many patients who have been treated with
Avastin but are not here to tell their stories.
Avastin will not disappear because of the F.D.A. decision. It remains available
for treating other cancers, and research to find its appropriate role in breast
cancer treatment continues. In the meantime, the F.D.A., which is expected to
make its decision in September, needs to resist Genentech’s attempt to have it
ignore scientific evidence.
Serious progress in the treatment of cancer will not be the result of polemics,
lobbying or marketing. Genentech’s money and efforts would be better spent on
research for more meaningful treatments for breast cancer.
Frederick C. Tucker Jr. is an oncologist.
Drugs and Profits, NYT, 24.6.2011,
http://www.nytimes.com/2011/05/25/opinion/25tucker.html
Our
Irrational Fear of Forgetting
May 21,
2011
The New York Times
By MARGARET MORGANROTH GULLETTE
Waltham,
Mass.
IN our hypercognitive society, fear of forgetfulness has made deep inroads into
the psyche. Misplacing car keys, once considered mere absent-mindedness, is now
a clinical symptom. Technological ineptitude in the prime of adulthood is
ascribed to memory failure.
The mere whiff of perceived memory loss can have terrible consequences in an
insecure economy in which midlife workers are regularly (and illegally) laid off
on account of their age. This epidemic of anxiety around memory loss is so
strong that many older adults seek help for the kind of day-to-day forgetfulness
that once was considered normal.
Greater public awareness of Alzheimer’s, far from reducing the ignorance and
stigma around the disease, has increased it. People over 55 dread getting
Alzheimer’s more than any other disease, according to a 2010 survey by the
MetLife Foundation. The fact that only 1 in 8 Americans older than 65 has
Alzheimer’s fails to register.
Is the prospect of the disease so horrifying that it should prompt someone to
consider suicide? A writer I know whose mother had Alzheimer’s told me she is
stockpiling pills. An academic told me he has found someone who will help him
die “before I lose my mind.”
Advocacy groups, manufacturers of so-called anti-aging products and the news
media have, for varying reasons, tended to inflate the number of sufferers and
the horrors of the condition. Doctors, too, have been complicit: some use
“cognitive impairment” as an argument for ending dialysis or other
life-sustaining treatments.
And some voices in our culture amplify these alarming sentiments. Tony Kushner
links Alzheimer’s to suicide in his new Off Broadway play, “The Intelligent
Homosexual’s Guide to Capitalism and Socialism With a Key to the Scriptures.”
His 72-year-old hero, Gus Marcantonio, a retired union organizer, tells his
assembled family that he has guessed he has Alzheimer’s, and wants to sell the
family house and kill himself over the weekend. Gus has no symptoms that the
audience can see except once losing his place in a voluble, earnest and moving
speech.
In the Korean director Lee Chang-dong’s film “Poetry,” which won the award for
best screenplay at Cannes last year, the graceful and empathetic heroine, who is
66, is given a diagnosis of Alzheimer’s. She too has no symptoms other than once
forgetting the word for “bus station.” Yet in the film she jumps off a bridge.
The characters have other motives besides fear to end their lives — guilt,
mainly. So why is Alzheimer’s brought into these plots so conspicuously? Perhaps
because no other motivation seems as plausible to an audience as a reason to
kill oneself.
Despite the prevalence of Alzheimer’s in our national conversation, diagnosing
the disease is actually difficult. There is no test that can predict whether
forgetting names or words like “bus station” is an indicator of the onset of a
degenerative disease. Many older people lose the ability to remember proper
nouns but then never progress to losing any other part of speech.
Most forgetfulness is not Alzheimer’s, or dementia, or even necessarily a sign
of cognitive impairment. And yet any prophecy about impaired cognition — whether
it is fulfilled or not — harms people’s sense of self. They begin to be treated
like children, patronized with baby talk or avoided. At the assisted living
facility where my mother lived until she died last year at age 96, the nursing
director told me that some people think Alzheimer’s is contagious. Victims of
misdiagnosis — or, just as devastating, self-diagnosis — dread being shunned,
rejected by their offspring, going into debt, becoming a “burden,” losing
selfhood.
It needn’t be this way. People with cognitive impairments can live happily with
their families for a long time. My mother was troubled by her loss of memories,
but she discovered an upside to forgetting. She had forgotten old rancors as
well as President George W. Bush’s name. We sang together. She recited her
favorite poems and surprised me with new material. We had rich and loving times.
Suicide didn’t cross her mind.
The mind is capacious. Much mental and emotional ability can survive mere memory
loss, as do other qualities that make us human.
In fact, a revolution in care-giving might be slowly taking root, at least among
those aware of alternative narratives of memory loss.
Thomas Kitwood, a British psychologist who was a pioneer in the field of
dementia care, died in 1998, but his books, which emphasize personhood instead
of debilitation, remain influential. “Making an Exit,” a memoir by Elinor Fuchs,
a drama professor at Yale, explored the conversational patterns of her mother
when she was in an advanced stage of Alzheimer’s. Anne Basting, director of the
Center on Age and Community at the University of Wisconsin, Milwaukee, who wrote
a play from poems created by people with Alzheimer’s, has a slogan: “Forget
Memory. Try Imagination.”
What a difference it would make if everyone began to share these attitudes. We
could make cognition-related fear-mongering shameful and rare, make debates
about end-of-life care less searing, improve treatment protocols, reaffirm our
collective compact with older people, ease our relationships with people of any
age who are cognitively impaired, and enable adults to look forward to getting
older with hope instead of despair.
Margaret
Morganroth Gullette, a scholar at the Women’s Studies Research Center at
Brandeis University, is the author of “Agewise: Fighting the New Ageism in
America.”
Our Irrational Fear of Forgetting, NYT, 21.5.2011,
http://www.nytimes.com/2011/05/22/opinion/22gullette.html
Health
Insurers Making Record Profits
as Many
Postpone Care
May 13,
2011
The New York Times
By REED ABELSON
The
nation’s major health insurers are barreling into a third year of record
profits, enriched in recent months by a lingering recessionary mind-set among
Americans who are postponing or forgoing medical care.
The UnitedHealth Group, one of the largest commercial insurers, told analysts
that so far this year, insured hospital stays actually decreased in some
instances. In reporting its earnings last week, Cigna, another insurer, talked
about the “low level” of medical use.
Yet the companies continue to press for higher premiums, even though their
reserve coffers are flush with profits and shareholders have been rewarded with
new dividends. Many defend proposed double-digit increases in the rates they
charge, citing a need for protection against any sudden uptick in demand once
people have more money to spend on their health, as well as the rising price of
care.
Even with a halting economic recovery, doctors and others say many people are
still extremely budget-conscious, signaling the possibility of a fundamental
change in Americans’ appetite for health care.
“I am noticing my patients with insurance are more interested in costs,” said
Dr. Jim King, a family practice physician in rural Tennessee. “Gas prices are
going up, food prices are going up. They are deciding to put some of their
health care off.” A patient might decide not to drive the 50 miles necessary to
see a specialist because of the cost of gas, he said.
But Dr. King said patients were also being more thoughtful about their needs.
Fewer are asking for an MRI as soon as they have a bad headache. “People are
realizing that this is my money, even if I’m not writing a check,” he said.
For someone like Shannon Hardin of California, whose hours at a grocery store
have been erratic, there is simply no spare cash to see the doctor when she
isn’t feeling well or to get the $350 dental crowns she has been putting off
since last year. Even with insurance, she said, “I can’t afford to use it.”
Delaying care could keep utilization rates for insurers low through the rest of
the year, according to Charles Boorady, an analyst for Credit Suisse. “The big
question is whether it is going to stay weak or bounce back,” he said. “Nobody
knows.”
Significant increases in how much people have to pay for their medical care may
prevent a solid rebound. In recent years, many employers have sharply reduced
benefits, while raising deductibles and co-payments so people have to reach
deeper into their pockets.
In 2010, about 10 percent of people covered by their employer had a deductible
of at least $2,000, according to the Kaiser Family Foundation, a nonprofit
research group, compared with just 5 percent of covered workers in 2008.
Doctors, for one, say patients’ attitudes are changing. “Because it’s from
Dollar 1 to Dollar 2,000, they are being really conscious of how they spend
their money,” said Dr. James Applegate, a family physician in Grand Rapids,
Mich. For example, patients question the need for annual blood work.
High deductibles also can be daunting. David Welch, a nurse in California whose
policy has a $4,000 deductible, said he was surprised to realize he had delayed
going to the dermatologist, even though he had a history of skin cancer. Mr.
Welch, who has been a supporter of the need to overhaul insurance industry
practices for the California Nurses Association union, said he hoped his medical
training would help him determine when to go to the doctor. “I underestimated
how much that cost would affect my behavior,” he said.
Dr. Rebecca Jaffe, a family practice doctor in Wilmington, Del., said more
patients were asking for the generic alternatives to brand-name medicines,
because of hefty co-payments. “Now, all of a sudden, they want the generic, when
for years, they said they couldn’t take it,” she said.
The insurers, which base what they charge in premiums largely on what they
expect to pay out in future claims, say they still expect higher demand for care
later this year. “I think there’s a real concern about a bounce-back, a rebound,
in utilization,” said Dr. Lonny Reisman, the chief medical officer for Aetna.
Because they say they expect costs to rebound, insurers have not been shy about
asking for higher rates. In Oregon, for example, Regence BlueCross BlueShield, a
nonprofit insurer that is the state’s largest, is asking for a 22 percent
increase for policies sold to individuals. In California, regulators have been
resisting requests from insurers to raise rates by double digits.
Some observers wonder if the insurers are simply raising premiums in advance of
the full force of the health care law in 2014. The insurers’ recent prosperity —
big insurance companies have reported first-quarter earnings that beat analysts
expectations by an average of 30 percent — may make it difficult for anyone,
politicians and industry executives alike, to argue that the industry has been
hurt by the federal health care law. Insurers were able to raise premiums to
cover the cost of the law’s early provisions, like insuring adult children up to
age 26, and federal and state regulators have largely proved to be
accommodating.
But 2014 and 2015 are likely to be far more challenging, as insurers are forced
to adjust to the law’s greatest changes, like providing coverage to everyone
regardless of whether they have an expensive pre-existing condition. “I think
they’re going to go through a winter,” said Paul H. Keckley, executive director
of the Deloitte Center for Health Solutions, a research unit of the consulting
firm Deloitte.
And while the slowing down of demand is good for insurers, at least in the short
term, the concern is that patients may be tempted to skip important tests like
colonoscopies or mammograms. The new health care law will eventually prevent
most policies from charging patients for certain kinds of preventive care, but
some plans still require someone to pay $500 toward a colonoscopy.
In recent times, insurers have prospered by pricing policies above costs, said
Robert Laszewski, a former health insurance executive who is now a consultant in
Alexandria, Va. The industry goes through underwriting cycles where the
companies are better able to predict costs and make room for profits. “They’re
benefiting from a very positive underwriting cycle,” he said.
“Maybe managed care is finally working,” he said. “Maybe this is the new
normal.”
Still, he emphasized, health care costs, even if they are rising at 6 percent or
7 percent a year, are increasing at a much faster pace than overall inflation.
“We haven’t solved the problem,” Mr. Laszewski said.
Health Insurers Making Record Profits as Many Postpone Care, NYT, 13.5.2011,
http://www.nytimes.com/2011/05/14/business/14health.html
Early
H.I.V. Therapy Sharply Curbs Transmission
May 12,
2011
The New York Times
By DONALD G. McNEIL Jr.
People
infected with the virus that causes AIDS are far less likely to infect their
sexual partners if they are put on treatment immediately instead of waiting
until their immune systems begin to deteriorate, according to preliminary
results from a large clinical trial released on Thursday.
Patients with H.I.V. were 96 percent less likely to pass on the infection if
they were on treatment — a finding that was so overwhelming that it is likely to
change the way American AIDS doctors treat patients and what treatment policies
are adopted by the World Health Organization and other countries, said Dr.
Anthony S. Fauci, head of the National Institute of Allergy and Infectious
Diseases, which paid for the trial.
The data was so convincing that the trial, which was due to last until 2015, is
effectively being ended early. Although participants will still be followed, all
will be offered antiretroviral drugs immediately. (Until now, those taking part
in the trial had been divided into two groups, one receiving treatment
immediately and the other after a delay.)
While there have been other studies that strongly implied that putting patients
on antiretroviral drugs early would prevent them from infecting others, this is
the first evidence from a randomized clinical trial, the gold standard in
medical research.
The $73 million trial, known as HPTN 052, involved 1,750 couples in 14 cities on
four continents. One member of each couple was infected with H.I.V.; the other
was not. In half the couples, chosen at random, the infected partner was put on
antiretroviral drugs as soon as he or she tested positive for the virus.
In the other half, the infected person started treatment only when his or her
CD4 count — a measure of the immune system’s strength — dropped below 250.
In 28 of the couples, the other partner became infected during the trial with a
strain of H.I.V. that was genetically proven to have come from his or her
partner. Twenty-seven of those 28 infections took place in couples in which the
infected partner was not yet getting treatment.
Early H.I.V. Therapy Sharply Curbs Transmission, NYT,
12.5.2011,
http://www.nytimes.com/2011/05/13/health/research/13hiv.html
A Social Networking Device for Smokers
May 10, 2011
The New York Times
By JOSHUA BRUSTEIN
Companies have started adding the ability to communicate wirelessly to an
increasing range of devices, like tablet computers, cars and refrigerators.
Now they are doing it with cigarettes.
Blu, the maker of electronic cigarettes that release a nicotine-laden vapor
instead of smoke, has developed packs of e-cigarettes with sensors that will let
users know when other e-smokers are nearby.
Think of it as social smoking for the social networking era.
“You’ll meet more people than ever, just because of the wow factor,” said Jason
Healy, the founder of Blu, who did not appear to be making friends as he exhaled
the odorless vapor of an e-cigarette at a coffee shop in Midtown Manhattan
recently. “It’s like with any new technology.”
E-cigarettes have several obvious advantages to their traditional counterparts.
They allow users to avoid bans on smoking in public places because they release
only water vapor. Mr. Healy and other e-cigarette manufacturers also claim that
they have practically no negative health effects — an assertion that draws
skepticism in many quarters. But the devices are also, in their own way,
gadgets.
The new “smart packs,” which will go on sale next month for $80 for five
e-cigarettes, are equipped with devices that emit and search for the radio
signals of other packs. When they get within 50 feet of one another, the packs
vibrate and flash a blue light.
The reusable packs, which serve as a charger for the cigarettes, can be set to
exchange information about their owners, like contact information on social
networking sites, that can be downloaded onto personal computers.
The packs also conveniently vibrate when a smoker nears a retail outlet that
sells Blu cigarettes.
Later versions will be tethered to a smartphone through an app, allowing more
options for real-time communication, Mr. Healy said. The company also plans to
develop a system through which the packs will monitor how much people are
smoking and report back to them — or to their doctors.
Marketers think people want more devices to link to each other. More than 105
million adult Americans have at least two types of connected devices, and 37
million have five or more, according to Forrester Research.
Nintendo’s new hand-held gaming systems, the 3DS, communicate with one another
when brought into close proximity. A smartphone app called Color allows users to
take photographs that are then automatically shared with anyone nearby who has
also downloaded the app. It recently raised $41 million from venture
capitalists.
But Charles S. Golvin, an analyst at Forrester Research who has studied
connected devices, said that ideas like Blu’s connected cigarettes or Color show
that digital connections can get ahead of the reasons for doing so.
“The way that groups of affinity are conferred just by physical proximity makes
a bit of sense,” he said. “If someone walks by with a Nintendo, great, I share a
common interest. The fact that I walk by a smoker? Seems like a weak link.”
Mr. Healy says he thinks the connected packs would be most useful in nightclubs,
where people are interested in striking up conversations and want to smoke
without being forced outside.
Adam Alfandary, 24, a Brooklyn resident who works for a technology start-up, was
skeptical. He said that the social aspects of smoking were a part of the reason
he continued to light up, but he scoffed at the idea of a cigarette that would
do the social part for him. “I think that’s the dumbest thing I’ve ever heard in
my life,” he said.
“And I’m saying that in full acknowledgment that smoking is one of the dumbest
things I can do.”
A Social Networking
Device for Smokers, NYT, 10.5.2011,
http://www.nytimes.com/2011/05/11/technology/11smoke.html
Alfred
Freedman, a Leader in Psychiatry, Dies at 94
April 20,
2011
The New York Times
By WILLIAM GRIMES
Dr. Alfred
M. Freedman, a psychiatrist and social reformer who led the American Psychiatric
Association in 1973 when, overturning a century-old policy, it declared that
homosexuality was not a mental illness, died on Sunday in Manhattan. He was 94.
The cause was complications of surgery to treat a fractured hip, his son Dan
said.
In 1972, with pressure mounting from gay rights groups and from an increasing
number of psychiatrists to destigmatize homosexuality, Dr. Freedman was elected
president of the association, which he later described as a conservative “old
boys’ club.” Its 20,000 members were deeply divided about its policy on
homosexuality, which its Diagnostic and Statistical Manual of Mental Disorders
II classified as a “sexual deviation” in the same class as fetishism, voyeurism,
pedophilia and exhibitionism.
Well known as the chairman of the department of psychiatry at New York Medical
College and a strong proponent of community-oriented psychiatric and social
services, Dr. Freedman was approached by a group of young reformers, the
Committee of Concerned Psychiatrists, who persuaded him to run as a petition
candidate for the presidency of the psychiatric association.
Dr. Freedman, much to his surprise, won what may have been the first contested
election in the organization’s history — by 3 votes out of more than 9,000 cast.
Immediately on taking office, he threw his support behind a resolution, drafted
by Robert L. Spitzer of Columbia University, to remove homosexuality from the
list of mental disorders.
On Dec. 15, 1973, the board of trustees, many of them newly elected younger
psychiatrists, voted 13 to 0, with two abstentions, in favor of the resolution,
which stated that “by itself, homosexuality does not meet the criteria for being
a psychiatric disorder.”
It went on: “We will no longer insist on a label of sickness for individuals who
insist that they are well and demonstrate no generalized impairment in social
effectiveness.”
The board stopped short of declaring homosexuality “a normal variant of human
sexuality,” as the association’s task force on nomenclature had recommended.
The recently formed National Gay Task Force (now the National Gay and Lesbian
Task Force) hailed the resolution as “the greatest gay victory,” one that
removed “the cornerstone of oppression for one-tenth of our population.” Among
other things, the resolution helped reassure gay men and women in need of
treatment for mental problems that doctors would not have any authorization to
try to change their sexual orientation, or to identify homosexuality as the root
cause of their difficulties.
An equally important companion resolution condemned discrimination against gays
in such areas as housing and employment. In addition, it called on local, state
and federal lawmakers to pass legislation guaranteeing gay citizens the same
protections as other Americans, and to repeal all criminal statutes penalizing
sex between consenting adults.
The resolution served as a model for professional and religious organizations
that took similar positions in the years to come.
“It was a huge victory for a movement that in 1973 was young, small, very
underfunded and had not yet had this kind of political validation,” said Sue
Hyde, who organizes the annual conference of the National Gay and Lesbian Task
Force. “It is the single most important event in the history of what would
become the lesbian, gay, bisexual and transgender movement.”
In a 2007 interview Dr. Freedman said, “I felt at the time that that decision
was the most important thing we accomplished.”
Alfred Mordecai Freedman was born on Jan. 7, 1917, in Albany. He won
scholarships to study at Cornell, where he earned a bachelor’s degree in 1937.
He earned a medical degree from the University of Minnesota in 1941 but cut
short his internship at Harlem Hospital to enlist in the Army Air Corps.
During World War II he served as a laboratory officer in Miami and chief of
laboratories at the Air Corps hospital in Gulfport, Miss. He left the corps with
the rank of major.
After doing research on neuropsychology with Harold E. Himwich at Edgewood
Arsenal in Maryland, he became interested in the development of human cognition.
He underwent training in general and child psychiatry and began a residency at
Bellevue Hospital in Manhattan, where he became a senior child psychiatrist.
He was the chief psychiatrist in the pediatrics department at the Downstate
College of Medicine of the State University of New York for five years before
becoming the first full-time chairman of the department of psychiatry at New
York Medical College, then in East Harlem and now in Valhalla, N.Y.
In his 30 years at the college he built the department into an important
teaching institution with a large residency program. He greatly expanded the
psychiatric services offered at nearby Metropolitan Hospital, which is
affiliated with the school and where he was director of psychiatry.
To address social problems in East Harlem, Dr. Freedman created a treatment
program for adult drug addicts at the hospital in 1959 and the next year
established a similar program for adolescents. These were among the earliest
drug addiction programs to be conducted by a medical school and to be based in a
general hospital. He also founded a division of social and community psychiatry
at the school to serve neighborhood residents.
With Harold I. Kaplan, he edited “Comprehensive Textbook of Psychiatry,” which
became adopted as a standard text on its publication in 1967 and is now in its
ninth edition.
During his one-year term as president of the American Psychiatric Association,
Dr. Freedman made the misuse of psychiatry in the Soviet Union one of the
organization’s main issues. He challenged the Soviet government to answer
charges that it routinely held political dissidents in psychiatric hospitals,
and he led a delegation of American psychiatrists to the Soviet Union to visit
mental hospitals and confer with Soviet psychiatrists.
After retiring from New York Medical College, Dr. Freedman turned his attention
to the role that psychiatry played in death penalty cases. With his colleague
Abraham L. Halpern, he lobbied the American Medical Association to enforce the
provision in its code of ethics barring physicians from taking part in
executions, and he campaigned against the practice of using psychopharmacologic
drugs on psychotic death-row prisoners so that they could be declared competent
to be executed.
In addition to his son Dan, of Silver Spring, Md., he is survived by his wife,
Marcia; another son, Paul, of Pelham, N.Y.; and three grandchildren.
Alfred Freedman, a Leader in Psychiatry, Dies at 94, NYT,
20.4.2011,
http://www.nytimes.com/2011/04/21/health/21freedman.html
Guidelines Allow Earlier Definition of Alzheimer’s
April 19,
2011
The New York Times
By PAM BELLUCK
For the
first time in 27 years, the definition of Alzheimer’s disease is being recast in
new medical guidelines that reflect fast-mounting evidence that it begins
ravaging the brain years before the symptoms of dementia.
The guidelines, to be issued Tuesday by the National Institute on Aging and the
Alzheimer’s Association, divide the disease into three stages: a phase when
dementia has developed, a middle phase in which mild problems emerge but daily
functions can still be performed, and the most recently discovered phase, in
which no symptoms are evident but changes are brewing in the brain.
“We’re redefining Alzheimer’s disease and looking at this in a different way
than had ever been done,” said Creighton Phelps, director of the National
Institute on Aging’s Alzheimer’s Disease Centers Program. “I think we’re going
to start to identify it earlier and earlier.”
The drive to diagnose Alzheimer’s before it has progressed into profound
dementia is also reflected in a bill introduced in Congress this month, which
would create specific Medicare cost codes for Alzheimer’s diagnosis, including
steps involving discussions between the patient’s doctor and caregivers, a
recognition that keeping family members well-informed can result in better
planning and care.
“Early diagnosis is really the key to this,” said Representative Edward J.
Markey, Democrat of Massachusetts and a sponsor of the bill. “Oftentimes family
members notice the symptoms in their loved ones, but it’s only years later that
they get diagnosed or understand what resources are available.”
The most striking addition to the guidelines concerns methods that assess brain
changes involved in Alzheimer’s, including brain scans and tests of cerebral
spinal fluid. Such methods measure what are called biomarkers, physiological
indicators that someone is likely to develop dementia eventually, just as
cholesterol and blood pressure are biomarkers of impending heart disease.
For now, the guidelines specify that Alzheimer’s biomarkers — including abnormal
levels of the proteins amyloid and tau, and shrinkage of certain brain areas —
should not yet be put into widespread use, but used only with patients enrolled
in clinical trials.
That is because scientists cannot yet standardize the results of the tests, or
know “what measure is truly abnormal and what measure is not,” said Marilyn
Albert, director of the Johns Hopkins Alzheimer’s Disease Research Center, and a
leader of one working group that developed the new guidelines.
As many as a third of people with amyloid plaques in their brains, for example,
have not developed Alzheimer’s symptoms by the time they die. The guidelines
also urge caution because there is currently no drug known to halt or
significantly delay the onset of symptoms, so people told they are likely to get
Alzheimer’s have no effective medication to take.
“We don’t have enough information about what to tell people,” said Dr. Steven
DeKosky, dean of the University of Virginia medical school, who participated in
one of the working groups. “Until you can tell a clinician, ‘If you do this test
you have X amount of reliability and to do that will make a difference in the
life of your patient’ — until then, it remains in the lab.”
But the guidelines reflect a sense in the medical community that the moment when
science will have more specific knowledge about biomarkers is not that far off.
They are intended to encourage more research so that drugs can be developed to
attack early brain changes and to identify people who might benefit from such
drugs when they become available.
The goal, said William Thies, chief medical and scientific officer for the
Alzheimer’s Association, is “extending the range of our ability to investigate
this disease and eventually find the treatment that is going to be so necessary
to avoid the epidemic of Alzheimer’s disease that we see facing us over the next
40 years.”
In the short term, the biggest impact is likely to be seen with people who fall
into the middle phase, those with mild cognitive impairment linked to
Alzheimer’s. Experts say there are at least as many people experiencing this
phase as the 5.4 million people estimated to have Alzheimer’s dementia. And they
expect others to now ask their doctors if they are showing signs of mild
impairment, which include experiencing some difficulty or inefficiency with
memory, attention or other mental faculties, while still being able to function
independently.
Dr. Albert said that if patients with symptoms of mild cognitive impairment
wanted to “increase the certainty” of the diagnosis by getting a brain scan or
spinal fluid test, they should obtain such tests in a research trial so they
have a better chance of getting accurate results.
The guidelines also clarify diagnosis criteria for people with dementia
symptoms, distinguishing Alzheimer’s from other dementias, including vascular,
fronto-temporal and Lewy body. And they note that the earliest symptom of
Alzheimer’s dementia is not always memory loss, but could be mood changes or
problems with language, spatial perception or reasoning.
Dr. Pierre Tariot, director of the Banner Alzheimer’s Institute in Phoenix, who
was not involved in drafting the guidelines, called them “a step in the right
direction” that he hoped would not be “misconstrued” as a sign that biomarker
tests are further along than they are. He added, “The notion that Alzheimer’s
disease is a continuum that has an extensive pre-symptomatic phase is a very
important message to get out.”
Dr. Phelps said it would hardly be the last word from the medical community on
Alzheimer’s.
“We’re not drawing a line and saying this is it,” Dr. Phelps said. “What we’re
saying is this is the best of our knowledge and we’re not going to wait 27 years
to revisit these again.”
Guidelines Allow Earlier Definition of Alzheimer’s, NYT,
19.4.2011,
http://www.nytimes.com/2011/04/19/health/19alzheimer.html
A New
Push to Let H.I.V. Patients
Accept
Organs That Are Infected
April 11,
2011
The New York Times
By PAM BELLUCK
David
Aldridge of Los Angeles had a kidney transplant in 2006, but he will soon need
another. Like many people living with H.I.V., he suffers from kidney damage,
either from the virus or from the life-saving medications that keep it at bay.
Until recently, such patients did not receive transplants at all because doctors
worried that their health was too compromised. Now they can get transplants, but
organ-donor waiting lists are long. And for Mr. Aldridge, 45, and other H.I.V.
patients, a potential source of kidneys and livers is off limits, because it is
illegal to transplant organs from donors who test positive for the virus — even
to others who test positive.
But federal health officials and other experts are calling for repeal of the
provision that bans such transplants, a 23-year-old amendment to the National
Organ Transplant Act.
“The clock is ticking more quickly for those who are H.I.V.-positive,” said Dr.
Dorry Segev, transplant surgery director of clinical research at Johns Hopkins
and a co-author of a new study indicating that 500 to 600 H.I.V.-infected livers
and kidneys would become available each year if the law were changed. “We have a
huge organ shortage. Every H.I.V.-infected one we use is a new organ that takes
one more person off the list.”
The ban on transplanting organs from people with the virus that causes AIDS was
passed at the height of the AIDS scare in 1988, when infection with the virus
was considered a death sentence. But now many people with H.I.V. are living long
enough to suffer kidney and liver problems, adding to the demand for organs.
This has led some health authorities to say that H.I.V.-infected organs should
be available for transplant, primarily for patients infected with the virus but
also potentially for some who are not.
The federal Centers for Disease Control and Prevention and other health agencies
are about to issue new guidelines that will encourage a first step: research
involving transplanting H.I.V.-positive organs into H.I.V.-positive people. That
would require the transplant ban to be lifted.
“We would like to see as many safe transplants occurring as possible, and
there’s no reason why H.I.V.-positive recipients shouldn’t get transplants and
that H.I.V.-positive donors can’t be used,” said Dr. Matthew Kuehnert, who
directs the C.D.C.’s Office of Blood, Organ and Other Tissue Safety.
“I could see someone saying: ‘That’s horrible. Why would you want to transplant
H.I.V.?’ ”he said. “They don’t understand. Anyone who understands transplant
today, in the current era, understands the need.”
The H.I.V. Medicine Association, a professional group, just issued a similar
statement, calling for “changing federal law on H.I.V.-infected organ donation.”
Its chairwoman, Dr. Kathleen Squires, said her organization and other medical
groups would lobby Congress this year.
Until recent years, H.I.V.-positive patients were not given transplants because
of concerns that the virus could destabilize transplanted organs or that the
immunosuppressive drugs used in transplants might make the virus more dangerous.
But a large clinical trial found that results in H.I.V.-positive recipients are
“just as good as H.I.V.-negative patients, more or less,” said the study’s
leader, Dr. Peter Stock, a transplant surgeon at University of California, San
Francisco. “Our kidney patients do slightly worse than the general population of
transplant patients, but better than kidney transplant patients over 65.”
Last year, at least 179 H.I.V-positive people received kidneys or livers, up
from 9 in 2000.
Allowing H.I.V.-positive organs to be used would create an additional supply
when some 110,000 Americans are awaiting transplants. They often wait years, and
sometimes are too sick when organs become available to benefit from them.
There are concerns, even among some supporters of changing the law.
“People I know in the gay community are very split on it,” said Michael Bauer,
45, of Iowa City, who became H.I.V.-positive two years ago and will probably
need a liver transplant in coming years. “There’s the concept that having an
H.I.V-positive donor could actually be more damaging. You could have a donor who
has a tougher strain of H.I.V.”
Doctors say this and other risks could probably be managed by screening out the
sickest donors and recipients. And for patients like Mr. Bauer, the risks may be
worth it.
“I can get slapped on a list for a healthy liver, but there’s a whole slew of
people ahead of me,” he said. “I don’t want to be excluded from options.”
Others fear that H.I.V.-infected organs could be transplanted by mistake. While
extremely rare, such errors have occurred.
In Chicago in 2007, four recipients were infected by organs from a single dead
donor; the body had tested negative, but the test was administered too early,
before the virus could be detected. In 2009 a kidney recipient in New York was
infected from a living donor, who tested negative, then had unprotected sex and
became infected in the 79 days before the transplant. That case prompted the
federal disease centers to issue stricter testing recommendations this year, and
Dr. Kuehnert said the new guidelines would address ways to make transplants even
safer.
Not all the consequences of transplants involving H.I.V. patients are understood
yet. Dr. Stock’s patients, for example, were two to three times as likely as
other recipients to begin rejecting their healthy donated kidneys. More
immunosuppressive drugs helped them adjust, he said, but the donated kidneys may
wear out sooner, necessitating additional transplants.
The only known transplants involving H.I.V-positive donors and recipients,
conducted in South Africa, have so far been successful.
There, with H.I.V. widespread, Dr. Elmi Muller, a Cape Town surgeon, performed
four transplants in 2008 — “instead of wasting these kidneys, throwing them
literally in the bin,” she said. After word got around, she said, some people
questioned “whether it was the right thing to do.”
Dr. Muller stopped while ethics committees reviewed the question, and she
ultimately obtained approval. Of 10 patients she has transplanted, only one has
experienced rejection problems. About 50 are on a waiting list.
In the United States, patients with hepatitis C, a disease many H.I.V-positive
patients also have, are now living with organs from donors with hepatitis C.
In 2004, Illinois passed a law allowing transplant of H.I.V-positive organs, and
“our hope was maybe other states will pick this up,” said Dr. Michael Abecassis
of Northwestern Memorial Hospital in Chicago. But federal transplant law
supersedes the state one.
If such transplants are allowed, they will most likely start with clinical
trials, and most organs will come from deceased donors; living donors are at
risk for liver and kidney problems themselves. Most recipients would probably be
H.I.V-positive because “we don’t really know what would happen to someone with
non-H.I.V. status,” Dr. Abecassis said.
But some experts, including Dr. Segev and Dr. Kuehnert, say they can foresee
such transplants even for H.I.V.-negative patients because contracting H.I.V.
would be preferable to kidney or liver failure.
“I don’t want to minimize living with H.I.V, but it is a medically treatable
disease now,” said Charlie Alexander, president of the United Network for Organ
Sharing, which manages the country’s organ transplant system. “In certain cases,
I think it would be medically appropriate.”
Mr. Aldridge, the Los Angeles patient, who has been H.I.V.-positive for 25
years, says he would certainly consider an infected kidney.
“There’s a stigma about transplanting us to begin with, with some people saying
why should an organ be quote unquote wasted on us,” he said. “So if we can help
each other it would make things much better for us. If I need a kidney
transplant to survive, then so be it.”
A New Push to Let H.I.V. Patients Accept Organs That Are
Infected, NYT, 11.4.2011,
http://www.nytimes.com/2011/04/11/us/11hiv.html
Newly
Born, and Withdrawing From Painkillers
April 9,
2011
The New York Times
By ABBY GOODNOUGH and KATIE ZEZIMA
BANGOR, Me.
— The mother got the call in the middle of the night: her 3-day-old baby was
going through opiate withdrawal in a hospital here and had to start taking
methadone, a drug best known for treating heroin addiction, to ease his
suffering.
The mother had abused prescription painkillers like OxyContin for the first 12
weeks of her pregnancy, buying them on the street in rural northern Maine, and
then tried to quit cold turkey — a dangerous course, doctors say, that could
have ended in miscarriage. The baby had seizures in utero as a result, and his
mother, Tonya, turned to methadone treatment, with daily doses to keep her
cravings and withdrawal symptoms at bay.
As prescription drug abuse ravages communities across the country, doctors are
confronting an emerging challenge: newborns dependent on painkillers. While
methadone may have saved Tonya’s pregnancy, her son, Matthew, needed to be
painstakingly weaned from it.
Infants like him may cry excessively and have stiff limbs, tremors, diarrhea and
other problems that make their first days of life excruciating. Many have to
stay in the hospital for weeks while they are weaned off the drugs, taxing
neonatal units and driving the cost of their medical care into the tens of
thousands of dollars.
Like the cocaine-exposed babies of the 1980s, those born dependent on
prescription opiates — narcotics that contain opium or its derivatives — are
entering a world in which little is known about the long-term effects on their
development. Few doctors are even willing to treat pregnant opiate addicts, and
there is no universally accepted standard of care for their babies, partly
because of the difficulty of conducting research on pregnant women and newborns.
Those who do treat pregnant addicts face a jarring ethical quandary: they must
weigh whether the harm inflicted by exposing a fetus to powerful drugs, albeit
under medical supervision, is justifiable.
“I’ve had pharmacies that have just called back and said: ‘This lady’s pregnant.
Why do you want me to fill this scrip? I can’t do that,’ ” said Dr. Craig Smith,
a family practitioner in Bridgton, Me. “But when you stop and think about what
actually happens during withdrawal and how violent it can be, that would
certainly be not in the baby’s best interest.”
Still, even doctors who advocate treating pregnant addicts have had moments of
doubt.
“At first I was going, ‘Gosh, what am I doing?’ ” said Dr. Thomas Meek, a
primary care physician in Auburn, Me. “ ‘Am I really helping these people?’ ”
There are no national figures that document the extent of the problem, but
interviews with doctors, researchers, social workers and women who abused
painkillers while pregnant suggest that it has grown rapidly, especially in
rural regions, where officials say such abuse is most common.
In Maine, which has been especially plagued by prescription drug abuse, the
number of newborns treated or watched for opiate withdrawal, known as neonatal
abstinence syndrome, at the state’s two largest hospitals climbed to 276 in 2010
from about 70 in 2005. Hospitals in states including Florida and Ohio reported
similar increases, and experts said the numbers were probably higher since
pregnant women are rarely tested for drug use and many mothers do not admit to
abusing opiates.
Tonya, 24, said she was introduced to painkillers like OxyContin, Percocet and
Vicodin while working the overnight shift at an industrial bakery an hour from
her home. Everyone — including co-workers, the boyfriend she met on the job and
their manager — was taking pills, she said.
“It was a lot easier to get through life and have energy,” Tonya said at Eastern
Maine Medical Center here in January, holding Matthew a month after his birth.
He was still being weaned off methadone.
Before she was pregnant, Tonya said, she quickly became addicted, spending all
of her money on pills bought on the street. She and her boyfriend, Josh, needed
to stave off withdrawal and get through the day, she said.
Now that she is in treatment, Tonya, who like most mothers interviewed for this
article did not want her last name used, said her focus was on Matthew. “We put
him in this situation,” she said, “and we have to help him out of it.”
‘How Little
We Know’
Rigorous studies on treating infant withdrawal are scarce, and the American
Academy of Pediatrics has not published guidelines since 1998.
“It’s really remarkable how little we know about the effect of prescription
drugs and even nonprescription drugs on the fetus,” said Dr. Nora D. Volkow,
director of the National Institute for Drug Abuse. “There are real roadblocks in
terms of helping us advance the field.”
Dr. Mark L. Hudak, a neonatologist in Jacksonville, Fla., is helping to revise
the pediatrics academy’s guidelines. “There are commonalities, but it’s not like
you can go to a Web site that says, ‘This is what should be used by everyone,’ ”
Dr. Hudak said. “No one knows what the best approach is.”
Within states, every hospital that delivers babies exposed to painkillers may
have its own approach. Eastern Maine treats affected newborns with tiny doses of
methadone, while Maine Medical Center in Portland uses morphine combined with
phenobarbital, a barbiturate that prevents seizures. Some hospitals are also
experimenting with clonidine, a mild sedative that can relieve withdrawal
symptoms.
There is growing debate over treatment for pregnant women addicted to
prescription drugs, in light of concerns over the effects on their babies. Many
are slowly weaned from their dependence with methadone, the standard of care for
decades. Methadone, when taken in prescribed doses, keeps a steady amount of
opiate in the body, preventing withdrawal and drug cravings that occur when
levels dip. But it, too, can be addictive and cause nagging side effects like
drowsiness. And for addiction treatment, it can be obtained only at federally
licensed clinics where most users have to report for a daily dose.
A growing number of addicts are instead taking buprenorphine, another drug used
to treat addiction that some studies suggest staves off drug cravings as
effectively as methadone but is less likely to cause withdrawal in newborns. In
rural areas of the nation, where methadone clinics are few, buprenorphine is
considered a promising alternative because it can be prescribed by primary care
doctors and taken at home.
But buprenorphine also appears not to work for some addicts.
Still, a study published in December in The New England Journal of Medicine
showed that babies whose mothers had taken buprenorphine required significantly
less medication after birth and less time in the hospital than did babies whose
mothers were treated with methadone. But researchers cautioned that exposure to
buprenorphine in utero can still cause withdrawal symptoms and that further
study was needed.
“We don’t want it misconstrued that buprenorphine is a miracle drug,” said
Hendrée E. Jones, a Johns Hopkins University researcher and the study’s lead
author.
Even less is known about longer-term effects on babies exposed to painkillers,
though in a second leg of their study, Dr. Jones and her fellow researchers plan
to follow the 131 babies in the cohort until they turn 3.
A recent study by the Centers for Disease Control and Prevention found that
babies exposed to opiates in utero, in this case legally prescribed painkillers,
had slightly higher rates of birth defects, including congenital heart defects,
glaucoma and spina bifida.
Experts say that since many drug users also smoke and abuse alcohol, not to
mention that they face extenuating circumstances like poverty, it is difficult
to tease out the effects of each substance on their offspring.
“Most of the literature suggests consistently that the drug exposure itself is
not the primary concern,” said Karol Kaltenbach, a professor at Jefferson
Medical College in Philadelphia who studies addiction in pregnant women. “It’s
the cumulative effect of the drug-using lifestyle — poverty, chaos in the home,
domestic violence. All those things affect development.”
Not all newborns exposed to opiates have severe enough withdrawal to need
medicine; at Maine Medical Center since 2003, about 55 percent of babies exposed
to buprenorphine and 80 percent of those exposed to methadone have needed
treatment. But it is hard to predict which ones will need it: a newborn whose
mother was on a high dose of either drug might need none, while a baby whose
mother took a low dose might experience acute withdrawal.
Babies known to have been exposed to drugs are often kept in the hospital for at
least five days because withdrawal symptoms usually do not set in immediately.
Nurses examine them for a checklist of symptoms every few hours, assigning each
baby a score that, if high enough, calls for treatment.
“They don’t stop crying, they can’t settle down, they don’t relax,” said
Geraldine Tamborelli, nursing director of the birthing unit at Maine Medical
Center, which in 2010 diagnosed opiate withdrawal in 121 newborns. “They’re
struggling in your arms instead of snuggling into you like a baby that is
totally fine.”
In the neonatal intensive care unit at Eastern Maine, Kendra, 3 days old, was
sleeping in a dark, silent room one morning, away from the bustle and bright
lights that can be especially irritating to babies going through withdrawal.
Nurses frequently crept in to observe her, though, and by the afternoon her
limbs had stiffened and she was crying excessively and having tremors; it was
enough to begin treatment.
“This seems to be ramping up fairly quickly for her,” said Dr. Mark Brown, the
hospital’s chief of pediatrics, “so the decision was to start treatment more
quickly.”
On the pediatric ward, Matthew started fussing while his mother, Tonya, talked
to reporters that afternoon in January; his cry had a strange, reedy pitch that
nurses say is common to babies with his condition. The small dose of methadone
he had received gave him gas and heartburn, for which he was given two stomach
medications. He also was on clonazepam, a muscle relaxant and anti-anxiety drug
that helped him metabolize the methadone more slowly.
Tonya said that at first she “didn’t believe in” methadone treatment during
pregnancy and that doctors had to persuade her that it would not hurt her fetus.
She had experienced wrenching withdrawal when she stopped using painkillers
after learning she was pregnant, she said, and the doctors had warned her that
“when I was feeling that bad, he was feeling 1,000 times worse.”
Tonya said that in a previous pregnancy, she quit using drugs altogether and
miscarried a month later.
“That was the last thing I wanted to happen this time,” she said.
Avoiding
Addicts, and Liability
Treating drug-dependent mothers and babies is often lonely work, with little
communication among the doctors who take it on. As Dr. Brown said, “My network
for people who do this is really very small.”
Dr. Mark R. Publicker, an addiction medicine specialist at Mercy Recovery Center
in Westbrook, Me., is on a mission to get more of the state’s doctors to treat
pregnant prescription drug abusers and more hospitals to deliver their babies.
Only a handful of doctors here treat pregnant women with buprenorphine, Dr.
Publicker said, partly because they fear liability and do not want to deal with
addicts.
The fact that most hospitals will not deliver the babies makes doctors even less
likely to treat the women.
“It’s mostly ignorance,” Dr. Publicker said. “It’s a concern that it’s a risky
proposition and that they’re going to wind up with an ill baby.”
In February, Dr. Smith persuaded Bridgton Hospital, which has only 25 beds, to
deliver the babies of women on buprenorphine — a major victory, he said, because
until then women in rural southwestern Maine had to drive an hour or more to
Maine Medical to deliver.
Courtney, a patient of Dr. Smith’s who discovered she was pregnant while in jail
for stealing OxyContin from her landlord, said buprenorphine treatment seemed
the best of her bleak options.
“I just don’t want to mess up,” she said.
Tonya, too, said she was determined to make things right for Matthew, who was
five weeks old when she took him home to a trailer outside Bangor. He is off the
methadone now and appears healthy, but Tonya still has to go to a methadone
clinic in Bangor every day for her dose and resist the pressures to return to
illicit drug use. Her boyfriend began using opiates as a young teenager, she
said, and his father and grandmother abused OxyContin along with him.
“I’m proud that I changed my life,” Tonya said. “But at the same time, when you
see your child in pain and you know your child is in pain because of a life
decision you made, it’s the hardest thing in the world.”
Newly Born, and Withdrawing From Painkillers, NYT,
9.4.2011,
http://www.nytimes.com/2011/04/10/us/10babies.html
Late
Clash on Abortion Shows Conservatives’ Sway
April 8,
2011
The New York Times
By JENNIFER STEINHAUER
WASHINGTON
— The emergence of abortion as the last and most contentious of the issues that
held up the budget deal reached Friday night highlighted the enduring influence
of social conservatives within the Republican Party even at a time when the Tea
Party movement’s focus on fiscal austerity is getting most of the attention.
The main abortion-related provisions sought by Republicans were stripped out,
apparently in return for deeper cuts in federal spending. But the intense push
by abortion opponents, including Representatives Christopher H. Smith of New
Jersey, Joe Pitts of Pennsylvania and Mike Pence of Indiana, sent a signal that
Republicans intend to keep social issues on the front burner as Congress moves
on to a further series of battles.
The abortion opponents lost on their effort to restrict money going to Planned
Parenthood and other abortion providers as part of the budget deal. But they
succeeded in winning agreement for a separate vote on that issue next week —
Senate Democrats are sure to defeat it — and in keeping in the budget deal a
provision that would restrict abortion financing in Washington.
The social conservatives established that they have a welcome ear in Speaker
John A. Boehner of Ohio, who has won awards from opponents of abortion rights
and during the debate over health care of provisions was a visible supporter of
preventing federal money from going to abortion providers like Planned
Parenthood. (Federal law already prohibits the use of federal dollars for
abortions..)
The main restrictions Republicans had sought were included in the first spending
bill passed by the House, in February. They had the backing, with varying
degrees of intensity, not just of Republicans identified primarily as social
conservatives but also of many fiscal conservatives. While the party ultimately
chose not to close down the government over its position on abortion, social
conservatives were heard more clearly on the issue than they have been since the
November election focused Washington on cutting spending.
“The life issue is important to a lot of us,” said Representative Steve Chabot
of Ohio, who has been very involved in anti-abortion measures in the past. “For
some, people, for example, abortion is more important. For some people, spending
is more important. For me, it would be hard to say one over the other.”
Republicans had sought to take away federal money for family planning for poor
women and give that money instead to states, to forbid the District of Columbia
from using its tax dollars to help the poor obtain abortions, and to end family
planning subsidies to some international groups.
In one sense, the flashpoint nature of the battle presented both parties with an
opportunity to energize their bases.
Senator Harry Reid of Nevada, the Democratic majority leader, stressed
repeatedly on Friday that his party was committed to defending abortion rights,
and he characterized the fight as one over women’s health. Equally, House
Republicans portrayed themselves as determined to stand by their principles.
But the high-profile fight held political peril for Republicans in particular
when it comes to appealing to women and the broad center of American politics.
In polls taken this year and last by The New York Times/CBS News, when Americans
were asked to name the most important problem facing the country, fewer than 1
percent cited abortion. In December, when respondents were asked how available
abortions should be to those who seek them, 36 percent said generally available,
40 percent said available with limits, and 20 percent said abortions should not
be permitted.
The risks were not lost on Republicans like Senator Susan Collins of Maine, who
favors abortion rights. “Senator Collins does not believe this rider belongs on
this bill,” said a spokesman, Kevin Kelley, in an e-mail before the deal was
announced. “She believes it is the height of irresponsibility for Congress to
jeopardize pay for our dedicated troops, who are serving in harm’s way in three
wars, because of a policy debate that can occur later this year.”
Other Republicans, including Senator Tom Coburn of Oklahoma, also urged the
party not to sacrifice the budget deal to make a point on the abortion issue.
Few Republicans wanted to be seen as shutting down the government over the
issue, which may be why House freshmen insisted that the issue was irrelevant to
the budget battle, even as aides to lawmakers negotiated them down to the wire.
“This is not about policy riders,” said Representative Raúl R. Labrador of
Idaho, echoing almost word for word seven other House Republicans and Mr.
Boehner as well. “It’s about spending.”
America has seen this play before. Over the nearly four decades since the
Supreme Court affirmed women’s abortion rights, Congress has worked to chip away
at them, often through measures like those on the table in the final stages of
the budget battle. Those efforts have largely been led by Republicans, but not
exclusively; it was Democrats who favored restrictions on abortion who came
close to unraveling the 2009 health care overhaul.
While the 87 freshmen Republicans in the House ran on a platform of containing
federal spending, and while some Republicans, like Gov. Mitch Daniels of
Indiana, have suggested de-emphasizing social issues until the nation’s fiscal
problems can be addressed, the desire among social conservatives to curb
abortion rights has never gone away.
While few of the measures Republicans sought would cut spending — in the case of
funds used by Planned Parenthood, it would simply move them — Republicans
repeatedly said supporting family planning groups was a waste of taxpayer funds.
“This has been an ongoing struggle for decades,” said Norman J. Ornstein, a
resident scholar at the American Enterprise Institute, a conservative research
group. “But in this particular context, there is a different twist. It is one
thing to be deeply opposed to a policy and look at every vehicle you can for
changing it. It’s another when you frame the entire narrative around the debt
crisis we face.”
Using the amendment process to pull away at abortion rights has a history that
dates back almost as far as Roe v. Wade, which was decided in 1973. In 1976, the
House passed the Hyde Amendment, which excludes abortion from health care
services provided through Medicaid.
The amendment has been tacked on to annual appropriations bills ever since.
Under the Balanced Budget Act of 1997, health maintenance organizations gained
the right to refuse to cover counseling or referrals for abortion on moral or
religious grounds. The law restricting the use of District of Columbia funds for
abortions, known as the Dornan Amendment, was first introduced in 1988.
In the final hour, another social policy amendment of sorts, one that would
finance a school voucher program in the District of Columbia near and dear to
Mr. Boehner, went into the bill.
Late Clash on Abortion Shows Conservatives’ Sway, R,
8.4.2011,
http://www.nytimes.com/2011/04/09/us/politics/09rider.html
W. H.
Prusoff, Who Developed AIDS Drug, Is Dead at 90
April 6,
2011
The New York Times
By WILLIAM GRIMES
William H.
Prusoff, a pharmacologist at the Yale School of Medicine who, with a colleague,
developed an effective component in the first generation of drug cocktails used
to treat AIDS, died on Sunday in New Haven. He was 90 and lived in Branford,
Conn.
The death was confirmed by his son, Alvin.
Dr. Prusoff spent most of his long career studying molecular derivatives of
thymidine, a building block of DNA. His work led him to develop two important
antiviral drugs.
In the early 1950s, he synthesized idoxuridine, a successful treatment for
infant keratitis. The condition, an inflammation of the cornea caused by the
herpes simplex virus, was the leading infectious cause of blindness. Idoxuridine
disrupted the virus’s ability to reproduce.
This was an important breakthrough. At the time, it was believed that antiviral
agents powerful enough to be effective would be too toxic for human use and that
those safe for use would be too weak to counteract a virus.
Idoxuridine overturned medical dogma and, after winning approval by the Food and
Drug Administration, became the first clinically used antiviral drug. For this
reason, Dr. Prusoff is sometimes called the father of antiviral chemotherapy.
In the mid-1980s, as the AIDS epidemic spread, Dr. Prusoff and a Yale colleague,
Tai-shun Lin, began looking at thymidine derivatives that had been developed to
treat cancer but discarded when they proved ineffective. One of these was
stavudine, also known as d4T, a molecular cousin of the first AIDS drug, AZT.
Both had been synthesized in the 1960s by Dr. Jerome P. Horwitz at the Michigan
Cancer Foundation, now the Karmanos Cancer Institute, in Detroit.
Dr. Prusoff and Dr. Lin resynthesized the molecule and found in laboratory tests
that it short-circuited the viral enzyme in H.I.V., causing it to produce short,
incomplete pieces of DNA rather than complete strands.
Yale took out a patent in the doctors’ names and licensed it to Bristol-Myers
Squibb for development. In 1992, it became the first drug to be tested under the
F.D.A.’s parallel-track policy, which allowed patients with life-threatening
illnesses to obtain drugs undergoing clinical trials.
After F.D.A. approval, stavudine was brought to market in pill form in 1994 and
sold under the brand name Zerit. It joined three other drugs, known as
nucleoside analogs, approved for treating H.I.V.: zidovudine (AZT), didanosine
(ddI) and zalcitabine (ddC). Eventually, these were joined by a new generation
of drugs known as protease inhibitors.
Because of its potential side effects, notably numbness, burning sensations and
loss of fat in the feet, legs or hands, the drug is now used primarily in poor
countries, where it is cheap and widely available.
Stavudine earned tens of millions of dollars for Yale each year — more than the
total amount for all its other licensed medicines combined. It also made
millions for Dr. Prusoff, who became a vocal supporter of a campaign initiated
by Doctors Without Borders to persuade Bristol-Meyers to lower the drug’s price
in sub-Saharan Africa, where AIDS was rampant.
In March 2001, the company announced that it was reducing the price of the drug
in Africa to 15 cents for a daily dose, from $2.23, and removing barriers to the
sale of generic equivalents there.
“We weren’t doing this to make money,” Dr. Prusoff told the Yale School of
Medicine Chronicle. “We were interested in developing a compound that would be a
benefit to society.”
William Herman Prusoff, known as Bill, was born on June 25, 1920, in Brooklyn.
His parents, Jewish immigrants from Russia, ran a small grocery.
He earned a degree in chemistry from the University of Miami in 1941. Rejected
by the Army because of his poor vision, he spent World War II inspecting fuses
at a munitions factory in Memphis and, as a health inspector, checking the water
supply and the kitchens in Miami Beach hotels where pilots were billeted.
Urged by his parents, he applied to medical school, without success. He later
enjoyed recalling that Yale deemed him so unqualified that it refunded his
application fee in a gesture of pity.
Instead, he earned a doctorate in chemistry from Columbia in 1949 and then
taught pharmacology in Cleveland at Western Reserve University (now Case Western
Reserve) before joining the pharmacology department at Yale in 1953.
Dr. Prusoff used some of his patent money to create the William H. Prusoff
Foundation, which supported numerous programs, including the Yale Initiative for
the Interdisciplinary Study of Anti-Semitism. He also endowed lectureships in
virology and pharmacology at Yale and several scientific prizes.
In addition to his son, Alvin, of Fairfield, Conn., he is survived by a
daughter, Laura, of Ortahisar, Turkey, and three grandchildren.
W. H. Prusoff, Who Developed AIDS Drug, Is Dead at 90,
6.4.2011,
http://www.nytimes.com/2011/04/07/health/research/07prusoff.html
Baruch
Blumberg,
Who
Discovered and Tackled Hepatitis B, Dies at 85
April 6,
2011
The New York Times
By H. ROGER SEGELKEN
Dr. Baruch
S. Blumberg, the Nobel Prize-winning biochemist and medical anthropologist who
discovered the hepatitis B virus, showed that it could cause liver cancer and
then helped develop a powerful vaccine to fight it, saving millions of lives,
died Tuesday in Moffett Field, Calif. He was 85 and lived in Philadelphia.
His family said he died, apparently of a heart attack, shortly after giving a
keynote speech at a NASA conference at the Ames Research Center in Moffett
Field, which is in the San Francisco Bay area. Dr. Blumberg had long been
associated with a NASA project to hunt for micro-organisms in space.
Dr. Blumberg’s prize-winning virology and epidemiology work began in the 1960s
at the Fox Chase Cancer Center in Philadelphia and took him and his colleagues
on field trips around the world, from Japan to Africa.
The work led to the discovery of the hepatitis B virus in 1967, the first test
for hepatitis B in the blood supply and the development in 1969 of the hepatitis
B vaccine — the first “cancer vaccine.” Dr. Irving Millman, a colleague at the
research center, was its co-creator.
Dr. Blumberg’s discoveries have been compared to those of Jonas Salk, the
developer of the polio vaccine. He shared the Nobel Prize in Physiology or
Medicine in 1976 with D. Carleton Gajdusek for their work on the origins and
spread of infectious viral diseases. (Dr. Gajdusek had discovered the cause of
the kuru, or “trembling disease,” prevalent in New Guinea.)
Almost 20 years later, after decades of hepatitis B-related studies and a global
search for medicinal plants to treat hepatic infections, Dr. Blumberg began what
he called his second career. In 1999 he became the founding director of the
National Aeronautics and Space Administration’s Astrobiology Institute.
The institute’s mission was to oversee research teams in the development of
life-detecting devices for planetary rovers and asteroid fly-bys, and to
scrutinize life forms in “extreme” environments on Earth, like the ocean bottom
and the geothermal cauldrons that produce geysers. He joined several expeditions
himself.
To these seemingly disparate endeavors — investigating disease-causing organisms
and postulating alien or primordial life forms — Dr. Blumberg contributed a
broadened understanding of the evolutionary phenomenon called polymorphism, in
which a species can adapt to an environment through changes in appearances and
functions.
From his base in Philadelphia, Dr. Blumberg began investigating viruses with a
study of yellow jaundice, so named because of the characteristic vivid yellowing
of the eyes and skin. As early as 1940, medical researchers had determined that
there were two different forms of virus-induced jaundice, one that is
transmitted as an intestinal infection, and the other spread mainly by blood
transfusions.
Scientific field trips to pinpoint the agent responsible for blood-borne
jaundice were conducted by Dr. Blumberg and his colleagues in the Philippines,
India, Japan, Canada, Scandinavia, Australia and Africa. Ultimately it was blood
serum from an infected Australian aborigine that yielded the so-called
Australian antigen, a protein found on the surface of the hepatitis B virus.
After he and Dr. Millman developed the hepatitis vaccine, they struggled to
interest a pharmaceutical company to help develop and produce it.
“Vaccines are not an attractive product for pharmaceutical companies in that
they are often used once or only a few times and they ordinarily do not generate
as much income as a medication for a chronic disease that must be used for many
years,” Dr. Blumberg wrote in an autobiographical essay for the Nobel committee.
Moreover, he said, the medical research community in the early 1970s remained
skeptical about the claim that a virus had been identified and a vaccine
developed.
Ultimately he and Dr. Millman signed an agreement with Merck & Company, whose
vaccine laboratories were near Philadelphia.
Dr. Blumberg’s discoveries are credited with saving millions of patients from
ever developing liver cancer. But in his scientific autobiography, “Hepatitis B:
The Hunt for a Killer Virus” (Princeton University Press, 2002), he observed
ruefully that hepatic disease was still killing 1.5 million people a year
worldwide — despite the widespread availability of the vaccines he helped
develop — and that 350 million were chronically infected.
Still, he was hopeful. “Life — and death — are full of surprises,” he wrote,
“and while it may be tempting fate to be too optimistic, it appears likely that
within the next few decades this virus will be effectively controlled.” (There
is still no vaccine for the blood-borne hepatitis C, one of the five known
hepatitis viruses.)
Dr. Blumberg’s traced his fascination with inherited variations in
susceptibility to disease to the volunteer service he did during medical school
at an isolated mining town in northern Surinam, where he delivered babies,
performed clinical services and undertook the first malaria survey done in that
region.
He was particularly interested in the sugar plantation workers who had been
imported from several continents.
“Hindus from India, Javanese, Africans (including the Djukas, descendants of
rebelled slaves who resided in autonomous kingdoms in the interior), Chinese,
and a smattering of Jews descended from 17th century migrants to the country
from Brazil, lived side by side,” Dr. Blumberg wrote in his Nobel essay. “Their
responses to the many infectious agents in the environment were very different.”
He wrote his first scientific paper based on these studies and would revisit the
tropics repeatedly. “Nature operates in bold and dramatic manner in the
tropics,” he wrote.
By the late 1990s Dr. Blumberg was immersed in astrobiology, as NASA called the
new science. Appointed by the NASA administrator, Dan Goldin, to lead the
Astrobiology Institute, Dr. Blumberg and his team were asked to address three
profound questions: How does life begin and evolve? Does life exist elsewhere in
the universe? And what is life’s future on Earth and beyond?
As in his disease studies, Dr. Blumberg sought collaborations with specialists
in a variety of fields, including physics, chemistry, geology, paleontology and
oceanography as well as biology and medicine that would “help us to recognize
biospheres that might be different from our own.”
While urging the development of instrumentation for astrobiological space
probes, Dr. Blumberg recommended equal efforts in the study of earthly
“extremophiles,” the organisms that somehow thrive in extreme temperatures,
pressures and chemical conditions.
In fissures in the deep ocean floor, Dr. Blumberg said, are extremophiles that
might resemble the earliest life forms on Earth or other planets. He described
Earth as “a place of extremes” during the first few hundred million years of its
4.5-billion-year existence, given to radical climate fluctuations, from searing
heat to immobilizing cold, amid constant meteorite bombardments and catastrophic
volcanic eruptions.
He speculated that life might have started on Earth at geothermal sites, either
underground or in the sea. The NASA venture — since diminished by administrative
changes and financing cutbacks — was welcomed by those who advocate a search for
extraterrestrial intelligence, known as SETI, and call the science “exobiology.”
Dr. Blumberg joined the board of the SETI Institute in Mountain View, Calif.
But in an interview with The New York Times in 2002, he said he would be “very
surprised if we found something in space, that it would look like E.T.”
“If we found something more like a virus or a bacteria,” he said, “that would be
astounding enough.”
Baruch Samuel Blumberg (Barry to his friends) was born in New York City on July
28, 1925, the second of three children of Meyer Blumberg, a lawyer, and Ida
Blumberg. After attending the Yeshiva of Flatbush in Brooklyn, he went to Far
Rockaway High School in Queens (whose graduates also include the Nobel
physicists Richard Feynman and Burton Richter).
His undergraduate studies at Union College in Schenectady, N.Y., were
interrupted by World War II, when he served as a Navy deck officer on landing
ships. Returning to Union College, he completed a bachelor’s degree in physics,
enrolled in graduate studies of mathematics at Columbia and transferred to
Columbia’s College of Physicians and Surgeons, earning his M.D. there in 1951.
Dr. Blumberg served a clinical fellowship at Columbia Presbyterian Medical
Center, went to Oxford University’s Balliol College for a doctorate in
biochemistry, and returned to the United States in 1957 to join the National
Institutes of Health, where he headed the Geographic Medicine and Genetics
Section until 1964.
Most of his research afterward was conducted at the Fox Chase Cancer Center. Dr.
Blumberg was also on the faculty of the University of Pennsylvania and its
School of Medicine as a professor of medicine, medical genetics and medical
anthropology.
Dr. Blumberg married Jean Liebesman, an artist, in 1954. She survives him, as do
two daughters, Anne Blumberg of Boston and Jane Blumberg of Oxford, England; two
sons, George, of Oxford, and Noah, of Chevy Chase, Md.; and nine grandchildren.
Dr. Blumberg saw his Nobel as more than an act of recognition. He said it helped
draw renewed attention to his work with enormously beneficial consequences.
After receiving the prize, he said, he was invited to China. “I spoke before
several thousand people,” he told The Times in 2002. “I provided them with a
copy of the patent, and now I’m told that it helped to change the direction of
what they were doing and led to the saving of a lot of lives.”
Saving lives, he said, was the whole point of his career. “Well, it is something
I always wanted to do,” he said. “This is what drew me to medicine. There is, in
Jewish thought, this idea that if you save a single life, you save the whole
world, and that affected me.”
Daniel E. Slotnik contributed reporting.
Baruch Blumberg, Who Discovered and Tackled Hepatitis B,
Dies at 85, R, 6.4.2011,
http://www.nytimes.com/2011/04/07/health/07blumberg.html
|